Big Data breathes Life into Next-gen Pharma R&D
In the pharma industry today, speed plays an important role as drug patent life is only 20 years and organizations have a short window to earn ROI. As a result, demands are enormous for innovative technology solutions that garner quick and smart results.
The process of drug development is lengthy and complex combined with several processes, applications and approvals. Unquantified and unstructured data is produced from multiple systems in various forms. However, recent advances in storage, network and computing technologies have enabled pharma companies to economically and efficiently harness this Big Data and turn it into a potent source of business strength.
This enables joint analyses of clinical and pre-clinical data from disparate sources and also lends transparency to translational research.
This paper attempts to explore how biological, chemical, clinical and non-clinical data will be collected and analyzed using Big Data analytics.
Introduction
Current challenges such as patent cliff combined with shrinking pipeline will drag pharma industry revenue down significantly. Pharma companies are now being under pressure to adopt groundbreaking drug technologies and enterprise-wide M&A to diversify the product portfolio to maintain revenue streams.
Conclusions drawn from typical clinical trials are now not adequate enough for drug value assessment and decision making. There is, therefore, a need for data-driven insights with real-world clinical evidence. Translational research along with comparative effectiveness is becoming imperative to understand a drug’s impact in real life.
We believe that only Big Data’s robust and scalable framework can support the huge volumes of data and maintain a balance in the four Vs - Volume, Velocity, Variety and Veracity.
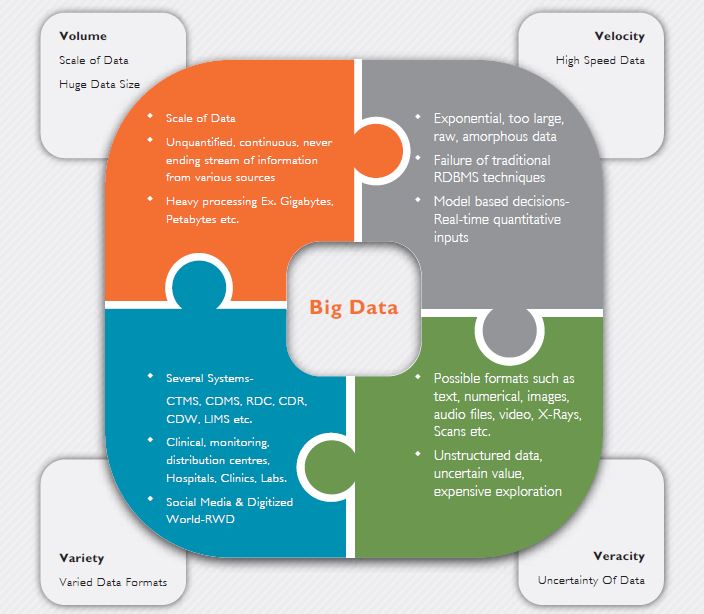
Figure 1: The four Vs
Implementation of Big Data infrastructure enables faster data processing, which, in-turn, allows organizations to support scientific analytics and derive more focused business outcomes for next-gen research.
Big Data architecture includes a radical integrated repository, along with scalable collaborative interfaces and advanced analytics with flexible deployment options.
Big Data architecture includes a radical integrated repository, along with scalable collaborative interfaces and advanced analytics with flexible deployment options. Big Data architecture includes a radical integrated repository, along with scalable collaborative interfaces and advanced analytics with flexible deployment options.
According to CBinsights, healthcare investments in Big Data totaled $274.5 million in 2012, and it went to $371.5 million in 2013.
Ref:- The CenterWatch Monthly, October 2013, A CenterWatch Article Reprint, Volume 20, Issue 10
Preparing for Next-Gen R&D
The current research is driven by securing regulatory safety and efficacy and mostly managed in silos. Focus is shifting towards real-world, integrated, connected research and care model, to support more complex disciplines.
The pharma industry is becoming more patient centric and realizing value of patient outcomes, improved safety and efficacy through better data insights. The data processing capabilities provided by Big Data and analytics ensure that insights are applied effectively.
In the traditional R&D model, internal and external generation of information was mostly managed in silos and separately validated for distinct analyses. Also there was significant professional and institutional misalignment between stakeholders such as biopharma, payers, providers and patients.
Whereas present integrated R&D model provides for more complex disciplines such as translational and evidence-based medicine, comparative effectiveness where stakeholders are largely aligned in their need to understand what interventions work for which individual patients and at what cost to offer better and economical care.
The new data processing and analysis paradigm such as Big Data technology is becoming inevitable for next-gen research.
Pharma companies would be interested in adapting next-gen research techniques by:
The next-gen R&D requires new outlook and ways of analysis to derive value from data generated from several stakeholders. Managing the entire data on a single, integrated platform is very essential in today’s world to make smart, cost-effective decisions to move faster in the value chain.
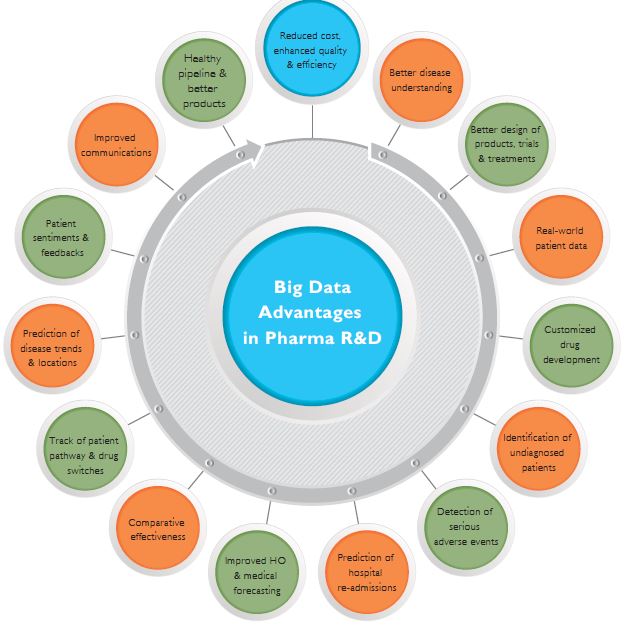
Figure 2: Advantages of Big Data in Pharma R&D
The growing transformation towards ”e” systems (e-CRF, ePRO and EDMS) is also making companies revisit their data management and analysis applications. With ”data-to-insights” cycle coming into play, pharma companies can emphasize more on a stack of tools such as Hadoop, NoSQL Databases,MapReduce, In-memory Analytics, Enhanced Cloud Computing and Storage. This will help organizations build a framework to extract useful features from large datasets to further understand how to model structured and unstructured data, and decrease the time to information.
The Way Forward
Pharma companies are likely to explore Big Data technologies for R&D efforts.
The technologies will help in:
Big Data technologies address the current data store issues and support specialized tools and facilities that can be used for normalizing and harmonizing data, used with advanced analytics for next-generation research.
The benefits of translational research can be viewed in Figure 3.
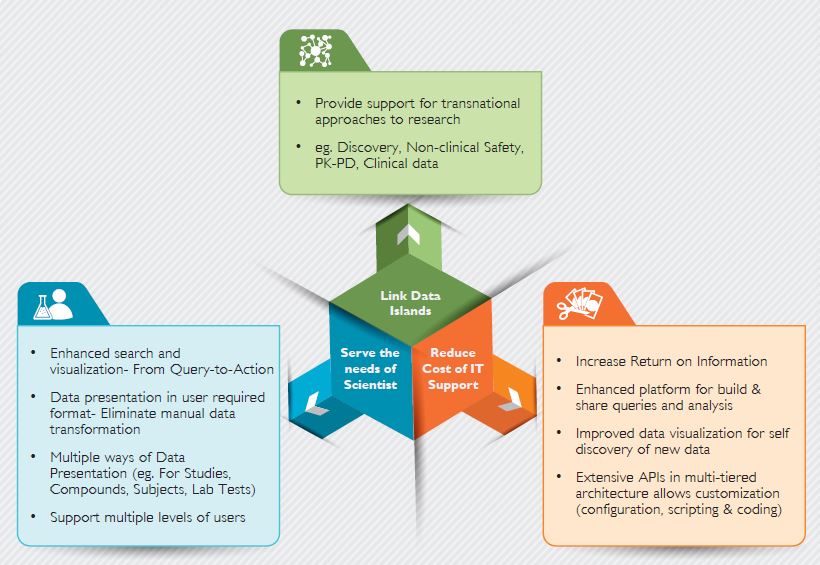
Figure 3: Benefits of Translational Research – Big Data Solution
This may benefit pharma companies by breaking the silos that separate internal functions and enhance collaboration with external partners extending their knowledge and data networks.
This will, in-turn, help the sponsors to quickly analyze their business and overall operations.
Conclusion
Integrated clinical research environment built on Big Data analytics platform facilitates next-generation research including detection and categorization of biomarker patterns, dose normalization, cross species modeling, trial simulations, similarity search and comparative effectiveness for real-world data.
Integrated Big Data analytics platform will link data islands, improve scientific support and regulatory decisions and enhance product safety. This will offer competitive advantage to pharma companies with faster time to market, and reduced IT support cost with innovations.
Simplifying the ”data-to-analytics” relationship represents the first step towards unifying Big Data and analytics approach, which is becoming a growth strategy for most of the pharma organizations.
References
Dr. Sarika Vanarse is a Principal consultant with Wipro’s Pharma R&D, Industry Solutions Group. She deals mainly with innovative R&D solutions development.
Dr. Vanarse holds a post-graduate degree in Medicine and has more than 11 years of Life Sciences IT consulting experience with Tier 1 organizations. She is currently leading several pharma R&D initiatives for large pharma companies.
Her expertise includes R&D strategy and innovation, pharma R&D intellectual property rights, R&D process optimization consulting, clinical informatics and analytics, and data integration.