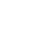Changes in the medical device regulatory framework, market globalization, and continuous innovation have led medical device companies to transition from legacy-driven business models to more compliant, competitive, and customer-oriented service models. Wipro’s medical services teams combine next-gen automation with deep industry expertise to deliver cutting-edge digital solutions across all our service areas.
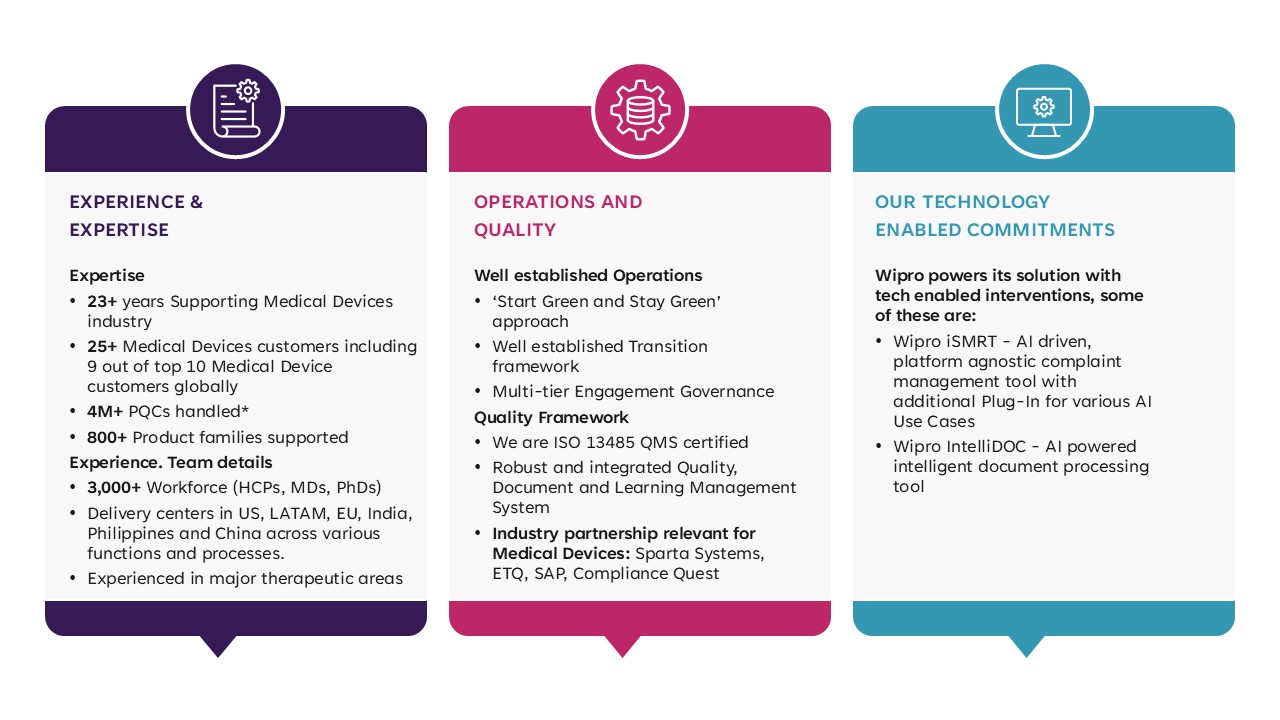
For over 20 years, Wipro has provided high quality medical device services to organizations across the world. Our global presence enables us to work closely with clients ranging from startups to large manufacturers and deliver tailored, localized solutions at competitive rates.
Wipro is ISO 13485 QMS certified and services a range of medical devices including software medical devices, consumer medical devices, home care devices, wearable and connected devices, drug device combination products and in vitro diagnostic (IVD) devices.