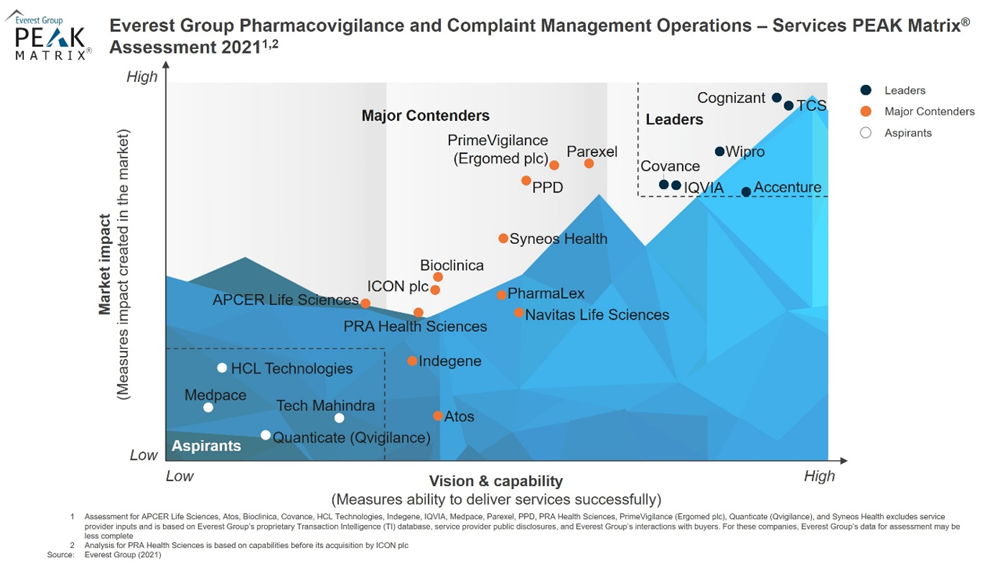
“With medical device regulations taking effect, having a standardized complaint handling process in place has become paramount for MedTech enterprises," said Manu Aggarwal, Vice President at Everest Group. "Wipro’s strong capabilities to support medical device manufacturers on post-market surveillance (complaint management) with scalable operations along with its growing pharmacovigilance portfolio has positioned it as a Leader in this year's Pharmacovigilance and Complaint Management PEAK Matrix® Assessment."
Wipro was positioned as a leader due to its strength on these important dimensions. A few are highlighted below:
- Wipro is one of the few service providers with capability to serve both BioPharma and MedTech enterprises with its strong pharmacovigilance / complaint management services. Key services include ADR/complaint intake (multi-lingual contact center capabilities), case processing / complaints investigation, and analysis and reporting.
- In addition to its growing complaint management business, Wipro has also been able to significantly increase its pharmacovigilance revenue in the last two years, growing at a CAGR of >30%.
- Wipro has a strong partnership ecosystem in place to augment its suite of services and technology within drug/device safety. Key partnerships include engagement with ComplianceQuest for quality management solution and Ontotext for literature screening.
- Wipro has made decent efforts in advancing its cognitive automation capabilities via its solutions – TaloSafe (an advanced version of its broad-based solution, Smartance) and other tools (such as Wipro-Medibot and CCI Agent) – to increase efficiencies and reduce overall operational costs.
- Wipro has developed a strong network of centers and balanced delivery mix across the geographies to deliver flexible services to its clientele.